U.S. panel will consider home AIDS test
A rapid home test for HIV, similar to early pregnancy tests, will be considered by a federal advisory committee on Tuesday, a move that many public health experts believe could eventually help calm Americans' fears of HIV, leading them to view it as just another serious chronic illness.
A rapid home test for HIV, similar to early pregnancy tests, will be considered by a federal advisory committee on Tuesday, a move that many public health experts believe could eventually help calm Americans' fears of HIV, leading them to view it as just another serious chronic illness.
An over-the-counter test offers new hope against an epidemic whose numbers in the United States have hardly budged in more than 15 years. An estimated 50 percent to 70 percent of the more than 50,000 new HIV cases annually are transmitted by people who were unaware that they were infected.
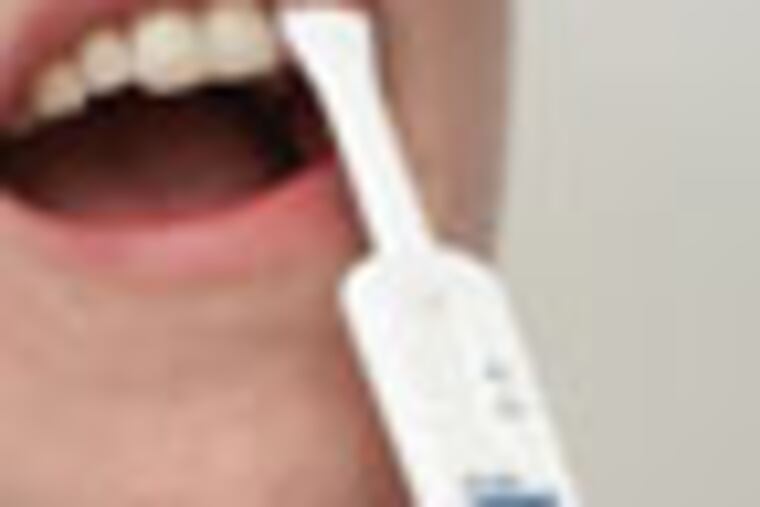
The screening test, made by OraSure Technologies Inc. of Bethlehem, Pa., relies on a simple swab of the gums. You insert the pad in a vial of fluid and wait 20 minutes. The appearance of two lines on the device indicates HIV, a preliminary result that must be confirmed by a laboratory blood test.
Even if the panel declines to endorse it, several experts said that the growing popularity of home health tests, coupled with the failure to get most people screened at clinics and doctor visits, mean that it is only a matter of time before a rapid HIV test is approved for use at home.
If the committee recommends the test on Tuesday, it would be the second historic action in less than a week against a disease that, while no longer a death sentence, still afflicts 1.2 million people. Another Food and Drug Administration advisory panel on Thursday signed off on giving an antiviral drug as a preventive measure to healthy people who are at high risk of exposure to the virus that causes AIDS.
Both moves would deploy existing technologies in new ways. The drug, a once-a-day pill called Truvada, is already approved for treatment of HIV. FDA approval would make it another form of prevention, along with safe sex and abstinence.
The OraSure test is already used by clinics worldwide to screen for HIV. This would add it to the drugstore aisle where condoms are sold.
"You don't have to stigmatize yourself and go to a doctor," said Jules Levin, executive director of the nonprofit National AIDS Treatment Advocacy Project. "You can walk into a pharmacy with sunglasses on. You can have someone pick it up for you."
Public health workers say that testing individuals ultimately protects the community.
"The only way we are going to control this epidemic is for people to know their HIV status and for people to act responsibly once they know their HIV status," said Marsha A. Martin, director of the Urban Coalition for HIV/AIDS Prevention Services, which represents health departments in the 10 cities, including Philadelphia, that are home to half of the nation's HIV positive population.
Based on evidence that most new cases are transmitted by people who were unaware that they were infected, the government has long focused prevention efforts on testing. That strategy was bolstered by research last year showing that starting treatment soon after diagnosis reduced the likelihood of infecting a partner by 96 percent.
Until a decade ago, HIV tests required blood to be sent to a lab. The only approved home test works that way: You lance a finger and mail in the blood drops. You call for results on the phone.
In 2002, OraSure triggered a major change when it introduced the first rapid blood-screening test. It allowed clinics to tell patients their status during a single visit; in the past, nearly a third never returned to get the lab results. The company added an oral swab version in 2004.
That product, the OraQuick Advance Rapid HIV-1/2 Antibody Test, is what OraSure wants permission to sell over the counter.
The test found 106 previously unknown infections during a clinical trial of more than 5,000 laypeople at 20 sites across the country. The company estimated that would translate to 9,087 cases for every one million tests by people in high-risk populations over and above current testing practices - and would therefore prevent more than 700 transmissions.
Sixty people were unable to use the test, for such reasons as spilled liquid (five) and inability to read instructions (four) or understand results (10).
It was 99 percent accurate overall, with one false positive and eight false negatives.
All of the false negatives were in high-prevalence populations. Those missed infections probably were too new to cause an immune response, said Nitika Pant Pai, a physician at McGill University in Montreal who has studied the test in settings around the world and believes it is appropriate for home use.
"This is a limitation of all antibody tests," she said. Antibodies aren't produced by the immune system for one to three months after infection, a highly contagious period.
Standard blood tests pick up signs of the virus earlier. They also are used to confirm positive screening tests.
"So what we need to do is educate patients," Pant Pai said, that they must get another test in three months if they engaged in risky behavior.
Since standard tests require a visit or phone call to find out results, professionals can discuss what they mean, talk about risks of unsafe sex, and link patients to treatment. Counselors can deal with emotional reactions.
None of that is guaranteed with a rapid home test.
"I think we really need to have a support system," said Raida Rabah, an infectious disease doctor in Chester County. She said a consumer HIV test, which would be the first infectious disease test approved for home use, might be an option for some people.
OraSure has promised round-the-clock live support from a toll-free call center that can link people to local treatment. Bilingual staff would be trained to help in a crisis but would refer people elsewhere for counseling.
Whether that is enough will likely be a key question - along with whether consumers can follow test instructions and get accurate results - for the FDA's Blood Products Advisory Committee on Tuesday. Its recommendation, like last week's approval of an HIV drug for prevention, then goes to the agency for a decision. The FDA typically follows its advisers' guidance but is not required to.
Arthur Caplan, a bioethicist at the University of Pennsylvania, said the questions should tip the balance against approval. As a method of controlling the epidemic, he said, a home screening test would be "about as helpful as owning a scale in your bathroom is in the war against obesity."
Another Penn professor, HIV prevention researcher David S. Metzger, had the opposite reaction. He cited recent treatment advances. "Now, knowing your HIV status and getting into care will save your life," he said.
So, who would buy the test?
"We are still figuring out the market," said Caroline Corner, an analyst for medical devices at MLV & Co., a New York investment bank. She estimated that the test would retail for about $50.
OraSure would not comment on price or likely sales but estimated the total U.S. market for the category at more than $500 million retail.
"The target market is really any sexually active adult ages 17 and older," said Douglas A. Michels, president and CEO of the 270-employee company.
Others said that those at the highest risk of HIV - homeless people, addicts, the poor - probably could not afford the test. They described two likely demographics that could: more affluent high-risk groups, such as gay men, who want frequent tests, and a broad population of Americans, some in suburban and rural areas, for whom the discomfort of visiting a clinic or asking a doctor has weighed more heavily than the need to know their HIV status.
"I feel like it is a good idea to get tested regularly," said Avir Mitra, 31, of Voorhees, who nevertheless has been tested only twice - once at a fair and, most recently, by his primary care doctor before starting medical school at Drexel University this year.
Mitra said he would pay $50 for a rapid test just to avoid waiting for lab results.
"It would be worth it," he said. "You get really paranoid about things like that: 'My god, what if I have AIDS?' "