Judge orders morning-after pill available for all ages
A federal judge in Brooklyn ruled Friday that emergency contraception must be available over the counter without restriction for women of all ages, giving the product the same retail status as cough drops and condoms.
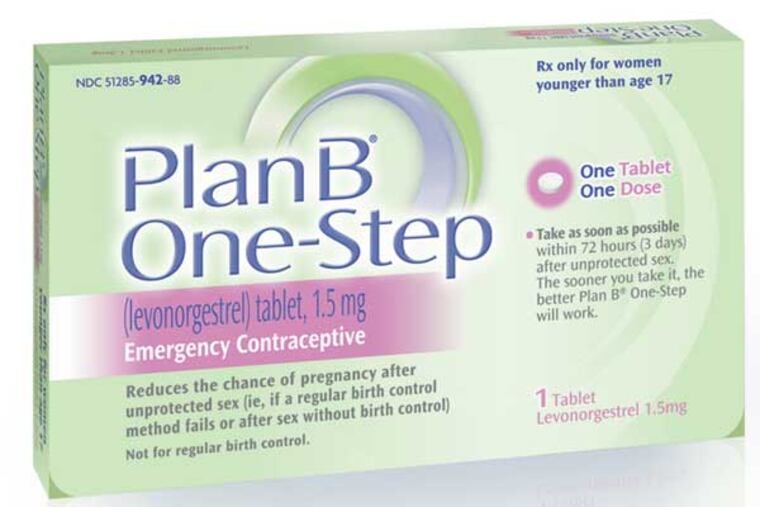
A federal judge in Brooklyn ruled Friday that emergency contraception must be available over the counter without restriction for women of all ages, giving the product the same retail status as cough drops and condoms.
U.S. District Judge Edward Korman's ruling caps a battle that began a dozen years ago when women's health activists first petitioned the U.S. Food and Drug Administration to make the so-called morning-after pill readily available without a prescription.
As he did in 2009, Korman on Friday assailed the FDA for delaying and defying the petition and subsequent lawsuit. But this time, he also blasted Health and Human Services Secretary Kathleen Sebelius for vetoing the FDA in 2011 when the agency was finally poised to remove restrictions on the backup birth-control pills.
Sebelius' decision was "politically motivated, scientifically unjustified, and contrary to agency precedent," Korman declared.
Emergency contraception contains a hormone used in birth-control pills and can be taken up to 72 hours after sex to reduce the chance of pregnancy.
Sold as Plan B One-Step and Next Choice, it is currently available without a prescription only to women 17 and older. The products are also kept behind the pharmacist's counter; purchasers must show a government-issued ID with proof of age.
Korman ordered an end to those limits within 30 days.
Reaction to the ruling was predictably polarized, reflecting the divide that has existed since advocates and the original manufacturer began pushing for over-the-counter sales.
Public health and medical groups called the ruling a victory for women's health, contending it will reduce unintended pregnancy and abortions. Conservative and antiabortion groups called it a blow to women's health, contending it will lead teenagers to have earlier, riskier sex, avoid consulting physicians, and become victims of sexual abuse.
"There is a real danger that Plan B may be given to young girls, under coercion or without their consent," Anna Higgins of the Family Research Council said in a statement.
Neither wonderful nor awful social effects have been substantiated, despite more than 1,000 studies of emergency contraception now in the medical literature.
Princeton University population researcher James Trussell now acknowledges that his early predictions of the method's impact were way too optimistic. In 1992, he and colleagues published a mathematical model that suggested making emergency contraception widely available could reduce abortions and unintended pregnancies in the United States by half.
But he still believes women should have the preventive option.
"This is a real victory for all women who want a convenient way to obtain emergency contraceptive pills," he said. It's "way overdue. Science finally trumps politics."
Plan B One-Step maker Teva Pharmaceuticals, an Israeli firm with U.S. headquarters in North Wales, Montgomery County, conducted safety studies requested by the FDA and cited by Korman in his ruling. But the company has consistently refused to discuss the legal battle or its sales plans.
The FDA also declined to comment, as did the U.S. Department of Justice, which has the option of appealing Korman's ruling.