More cases of lymphoma caused by breast implants, FDA says
At least 457 women in the U.S. have been diagnosed with anaplastic large cell lymphoma since 2010, including nine who died, the FDA said Wednesday.
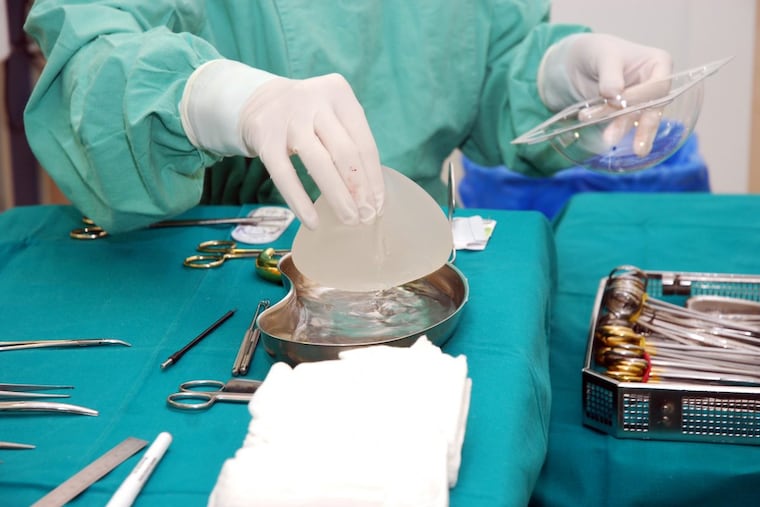
The number of cases of a life-threatening immune cell cancer caused by breast implants continues to rise, according to the latest tally of reports to the U.S. Food and Drug Administration.
At least 457 women in the United States have been diagnosed with anaplastic large-cell lymphoma since 2010, including nine who died, the FDA said Wednesday. That total has steadily climbed each year since 2011, when the FDA first issued a warning and said it had received 64 reports of the cancer.
Although most cases have involved textured-surface implants, some reports did not include information about implant surface, the FDA said. Smooth implants make up about 87 percent of the U.S. market.
Worldwide, at least 570 cases of the lymphoma, including 16 deaths, have been reported, according to the American Society of Plastic Surgeons.
For the first time, the FDA also said it has sent a letter to health care providers urging them to tell patients about the rare cancer, and report any suspected cases to the agency.
The FDA’s annual update comes as French health regulators prepare to meet this month to discuss the safety of textured implants, which account for 85 percent of the French market. In December, France’s National Agency for the Safety of Medicines and Health Products suspended its stamp of approval for Allergan’s textured implants and asked the company to recall its products from the European market. Allergan complied with the request, while defending the safety of its products.
“We are aware that our counterparts in different countries are taking certain actions or may be reporting different information about breast implant safety,” the FDA said in Wednesday’s statement.
Variation in markets and regulations make it difficult “to compare data and determine risk rates on a global scale,” the FDA said.
The agency plans to hold a meeting on March 25 and 26 to discuss implant lymphoma and new information on the safety of implants in general. The meeting is partly in response to women who have pushed for such a review, contending that their implants have caused a range of illnesses.