These 8 diseases are so rare that drug firms haven’t tried treating them with gene therapy. A $97 million program aims to help.
Gene therapy for one disease, Morquio syndrome, will be tested at Nemours Children's Health in Wilmington. Others will be tested at CHOP and the University of Pennsylvania.
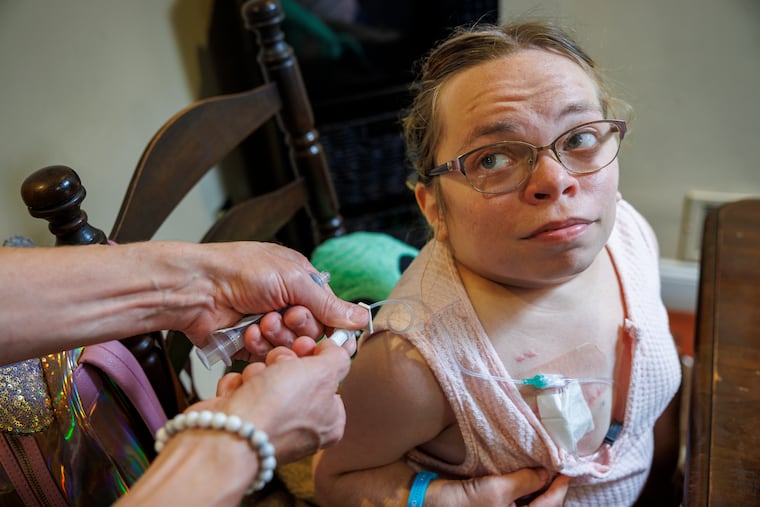
Every Wednesday, a nurse connects Sarah Van Orden to a tube for seven hours, infusing her with crucial enzymes that her body cannot make because of a genetic mutation.
In theory, the Lancaster, Pa., woman could avoid the weekly ordeal with a one-time treatment of gene therapy, an approach that has now been approved by the FDA for several other rare diseases.
But Van Orden’s disorder, a version of a condition called Morquio syndrome, occurs in just 1 in 250,000 births. The disease, which causes dwarfism and a host of other debilitating symptoms throughout the body, is so rare that gene-therapy companies are not interested in tackling it.
A new initiative aims to remove that hurdle, providing $97 million in funds and expertise to help researchers test gene therapy in patients with Morquio and seven other ultrarare diseases as soon as next year. The program, a partnership of nonprofits, government, and industry, is led by the nonprofit Foundation for the National Institutes of Health.
The Morquio trial will be conducted by researchers at Nemours Children’s Health, in Wilmington, where Van Orden, 26, has been traveling since childhood for treatment. Gene therapy for two of the eight other conditions will be tested at Children’s Hospital of Philadelphia and the University of Pennsylvania.
In addition to impeding her bone growth, Van Orden’s condition also has caused heart disease, impaired vision, difficulty breathing, and hearing loss.
Her enzyme treatments help alleviate the symptoms, but it is a challenge to spend seven hours every week hooked up to a tube.
“The idea of having potentially one gene therapy and then never having to do it again would be life-changing for me,” she said.
» READ MORE: Jim Wilson warns of gene therapy 'paradox' that deters investment
Researchers at Nemours have proposed testing gene therapy in 12 people with Morquio, including 10 children and two adults. But first, they must spend months finalizing the study protocol and other preparations, making use of the funds and expertise from the public-private partnership.
Conducting the study would be difficult without that boost, as it is not economical for one biotech firm to fund a gene therapy trial for an ultrarare disease by itself, said Nemours physician-scientist Shunji Tomatsu.
“A company cannot conduct this type of gene therapy trial unless the disease has a much higher frequency,” he said.
A costly drug
When Van Orden was an infant, her mother, a nurse, noticed that her rib cage was a bit flared out at the bottom, giving her chest a slight bell shape. A pediatrician told the family that there was no cause for concern.
But the flared-out ribs did not go away, and when Van Orden turned 2 ½, another physician told her parents she might have a type of skeletal abnormality.
Doctors at Nemours confirmed that hunch. She had the “A” form of Morquio, meaning she was unable to make an enzyme involved in recycling a type of sugar molecule.
The result was a toxic buildup of those molecules, both in her bones and in other cells throughout her body. Doctors said she might not live past age 20.
Van Orden underwent nine surgeries at Nemours, including the fusion of neck vertebrae to prevent spinal cord damage, and she has now beaten that prognosis by six years.
With the help of her weekly enzyme treatments — approved by the FDA when she was 14 — she expects to live a lot longer. The drug has improved her breathing ability and also has reduced the size of the abnormally thick mitral valve in her heart, improving its function.
But the treatment is not cheap. Called Vimizim, it costs more than $1 million a year for patients with Medicare or Medicaid, according to 2021 figures.
Gene therapy is expensive, too, often priced in the low millions. But it requires just one dose. If such a treatment were to work in Morquio syndrome patients, it could, in theory, enable them to avoid a lifetime of the expensive weekly infusions.
A standardized platform
That’s the goal of the $97 million project led by the Foundation for the National Institutes of Health, which is not part of the NIH but was chartered by Congress to support the agency’s mission. The project total includes cash and in-kind donations from 11 NIH institutes and centers, 12 life-science companies, and 10 nonprofit organizations.
Morquio and the seven other diseases were picked for a variety of reasons, said Courtney Silverthorn, the foundation’s associate vice president for science partnerships. The conditions all have been extensively studied by academic researchers, with animal models suggesting that gene therapy could be beneficial. Yet they are so rare — occurring in at most a few thousand patients worldwide — that gene-therapy firms have not pursued them.
“We wanted to identify opportunities that had a high unmet need,” she said.
The goal is to streamline and standardize the process of testing the genetic therapies in humans, so that researchers don’t need to start from scratch each time, Silverthorn said.
So for each of the eight diseases, physicians will deliver the beneficial genes into patients’ cells by using the same type of vector — a harmless, inactivated virus called an adeno-associated virus.
The foundation also is working with the eight research teams to standardize the dosing of the therapies, the metrics for quality control in manufacturing, and how the patient outcomes are measured.
“While we have eight diseases in the portfolio, we really hope that the work that we do will make every gene therapy for a rare disease more accessible,” she said.
The seven other conditions include rare neurological and retinal disorders. The gene therapy to be tested at Penn is aimed at a rare form of blindness, caused by a mutation in a gene called NPHP5. The one at CHOP is for a condition called multiple sulfatase deficiency, which can cause skeletal deformities and seizures.
If the gene therapy results are promising for any of them, researchers would then be free to pursue further studies with a commercial partner, Silverthorn said.
Earlier is likely better
While Van Orden is intrigued by the promise of gene therapy, she is not yet sure if she wants to volunteer to be one of the two adults in the Nemours study next year.
That’s because she would have to stop getting the weekly enzyme infusions — which have helped her — so that doctors could determine if the gene therapy enabled her own cells to make the enzyme.
Tomatsu, the lead Nemours researcher, says that gene therapy is likely to be more effective in young children, before the widespread progression of symptoms. The reason for including two adults in the study is partly to help demonstrate the safety of the treatment, he said.
Once the disease is diagnosed, typically in early childhood, the complications can be severe.
In Van Orden’s case, the fusion of her neck vertebrae required two surgeries. Surgeons also straightened her misaligned knees and fashioned hip sockets for her, because she was born without them. (As a result, she is able to walk without assistance, and works as a clerk in the Lancaster County treasurer’s office.)
Van Orden got her last surgery at age 13, but the enzyme infusions continue. Every Wednesday, a nurse comes to her house to administer it, with the assistance of Van Orden’s mother, Ruth, who also is a nurse.
“It’s a long day,” Sarah Van Orden said.
If she tries the gene therapy, the weekly visits could become a thing of the past. But even if she sticks with the infusions, she knows the enzyme has been the key to good health.
“It’s been a game changer,” she said. “It saved my life.”