Philly discovery for kids with leukemia prepares to go global
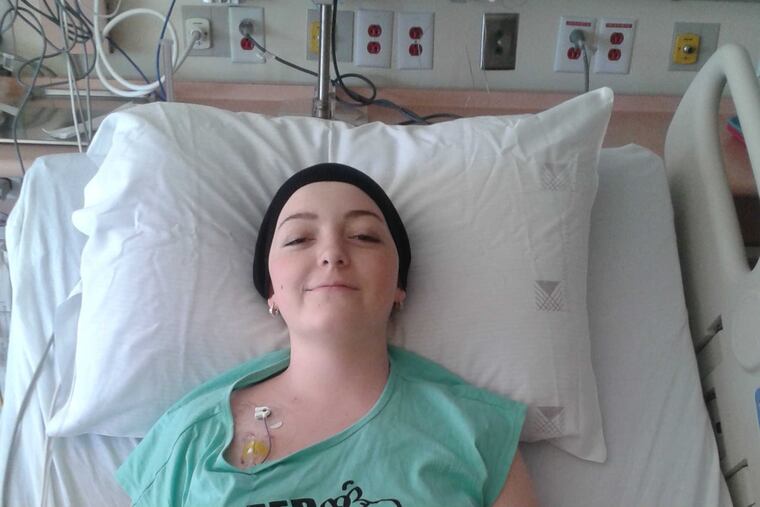
Fifteen months ago, 15-year-old Emma Collins was dying of leukemia. With no other option, her parents took her from their home in rural Canada for a last-ditch experimental treatment at Children's Hospital of Philadelphia, a choice that families from Croatia and even beyond have made.
Now, Philadelphia researchers and Novartis have shown they can deliver the complex, personalized, bioengineered therapy that saved Emma's life to desperately ill children around the world, rather than requiring them to make the journey to Philadelphia.
Initial results from the first-ever international clinical trial of the T-cell treatment, presented recently at the American Society of Hematology meeting, put the pharmaceutical giant far ahead in the high-stakes race to market the first such immunotherapy. The company said it will file early next year for U.S. Food and Drug Administration approval of the pediatric leukemia therapy, and later in 2017 for European regulatory approval.
Twenty-five highly specialized medical centers in North America, Europe, Japan, and Australia are part of the ongoing trial. Of the first 50 children treated, 41, or 82 percent, had no signs of acute lymphoblastic leukemia three months later. At six months, 24 children remained cancer-free. Those results parallel the high rates of lasting remissions achieved over the last four years in nearly 150 children treated at CHOP.
The logistical feat of the global trial, in contrast, was unparalleled. Each child's T cells were collected at the participating hospital, frozen, and shipped to Novartis' new cellular manufacturing plant in Morris Plains, N.J. Once there, the cells were thawed, genetically manipulated and multiplied over three weeks, refrozen, and sent back to be thawed and given intravenously to the child. Only three patients' cells could not be grown, a 6 percent "manufacturing failure" rate that was lower than the researchers expected.
"We are certainly very proud of the achievement," said Samuele Butera, Novartis' global leader of cell and gene therapies. "Assuring supply in all these parts of the world was definitely a challenge."
CHOP pediatrician Stephan Grupp, who led the far-flung experiment, said the prospect of having the world's first approved T-cell immunotherapy "is the most exciting thing I've been involved with in my life as a scientist, and especially as a clinician."
Novartis' ability to make individualized living products on an assembly-line scale should reassure some skeptical industry observers. Still, the economics of anti-cancer T cells remain uncharted because the technology is so expensive; analysts say the price tag could be as much as $500,000 per patient. Plus, it has not yet worked well in solid cancers other than melanoma.
Pediatric lymphoblastic leukemia would be a niche market. In the United States and Europe, about 6,000 cases are diagnosed each year, and 85 percent are cured with current standard therapies -- including bone marrow transplants, which also cost about $500,000, but are far more widely available and better studied. Butera said Novartis hopes the T-cell treatment will eventually be approved for an adult blood cancer called diffuse large B-cell lymphoma. That still would not be blockbuster territory.
Getting approval for pediatric leukemia "is more of a boutique indication," Grupp said. "The question is always going to be: How can we expand this to other cancers?"
Michael Rice, an oncology analyst with Defined Health, a health-care investment consulting firm, said Novartis' manufacturing tour-de-force validates the T-cell approach, but being first to market may not be an automatic advantage.
"It's been an intense race to get to market with the first product, but I'm not sure if the first products will be the ones that will remain," he said. "It will be a continual process of improvement as we figure out what works best, not just in terms of the effectiveness but also the toxicities."
One type of toxicity that has stymied T-cell use in solid cancers is called "off-target effects:" The killer T cells are programmed to target a distinctive tumor cell marker that turns out to be present on healthy cells. Leukemia and other blood malignancies that arise from "B cells" can be safely targeted, but even so, other types of toxicity have been a problem. Juno Therapeutics, a leading contender in the T-cell race, last month suspended a trial in lymphoma patients after two suffered brain swelling and died. It was the second time in six months that the trial had been suspended because of patient deaths.
"Obviously, every company has a different technology," Butera said. "As for Novartis, we have not seen any deaths. We haven't seen any cerebral edema."
However, Novartis' version, pioneered by University of Pennsylvania scientist Carl June, has consistently caused patients' immune systems to become over-stimulated as the T cells launch their attack.
As Emma Collins' case shows, the potential for complications and the need for monitoring are more reasons that offering the therapy closer to patients' homes would be a huge benefit.
The Collinses, who live in Muskoka, Ontario, spent six weeks in Philadelphia, far away from their teenage son, while Emma's cells were engineered and then given intravenously to her in October 2015.
Four days after the infusion, her cancer was gone.
But when the Collinses returned to CHOP three months later, in January 2016, tests showed the T cells were dying.
Emma received another T-cell infusion. This time, she suffered an extreme immune system overreaction. "She had a fever of 111. She was almost in a coma," her father recalled. "But I guess the cells 'took' better."
Emma has been in remission for almost a year now. She is again a standout on her high school volleyball and basketball teams. And her parents are thrilled that the therapy is moving toward wider availability. If Emma could have waited longer for the T-cell treatment, she might have been able to get it at the Hospital for Sick Children in Toronto -- a two-hour drive from her home. That hospital is part of the global trial.
"When I look back, and remember her so thin, no hair, in a wheelchair, and then I fast forward to now, it's just amazing," her father said. "She's back to normal. She's thriving."
In the global trial, 82 percent of the children had at least mild immune overstimulation, and 45 percent had severe symptoms like Emma, including very high fevers, low blood pressure, delirium, seizures, and liver abnormalities. They required anti-immune drugs and intensive care.
For parents of children who are facing death, the risk of overstimulation pales in comparison to the potential benefit of the T-cell therapy -- a cure.
"I personally avoid that word," Grupp said. "I talk about it in terms of 'long-term disease control.' That includes the first patient we ever treated, who is doing very well."
That's Emily Whitehead of Philipsburg, Pa. More than four years after her Lazarus story made international headlines, the 11-year-old and her parents are tireless advocates and fund-raisers for T-cell therapy research.