Novartis’ T-cell therapy for blood cancer, developed with Penn, wins European approval
The European Commission has followed the U.S. Food and Drug Administration in approving a genetically engineered T-cell therapy for certain blood cancers. The European price tag remains to be seen.
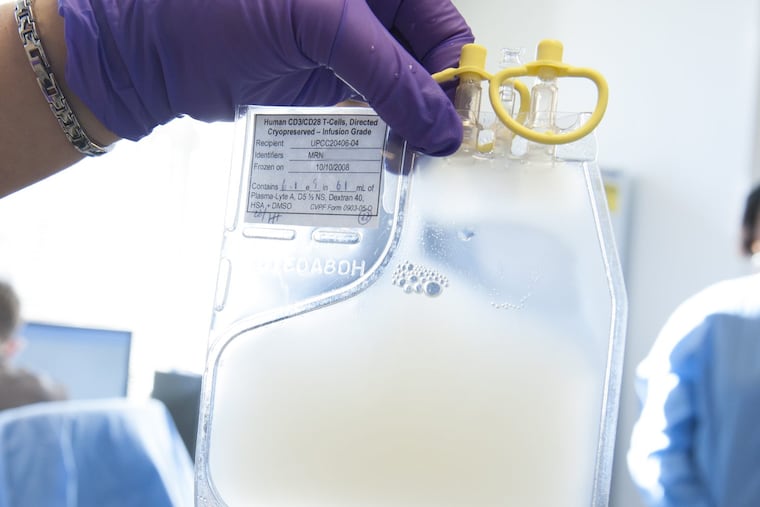
Novartis on Monday announced European approval for Kymriah, the groundbreaking T-cell therapy for blood cancer developed with the University of Pennsylvania and Children's Hospital of Philadelphia.
However, availability in each country will depend on reimbursement, manufacturing, and clinical training issues that the company is still working out.
Kymriah, or tisagenlecleucel, became the world's first T-cell therapy with the initial approval by the U.S. Food and Drug Administration a year ago for an advanced pediatric blood cancer called acute lymphoblastic leukemia. In May, U.S. approval was expanded to an aggressive adult disease called diffuse large B-cell lymphoma. The European Commission approval is for both cancers.
A one-time treatment, Kymriah is made by siphoning each patient's T cells, genetically programming these immune soldier cells to attack malignant blood cells, then returning them to the patient.
While the immunotherapy is offering new hope to patients who are out of treatment options, the jaw-dropping U.S. list prices have been controversial. The leukemia treatment costs $475,000 plus hospital costs (although reimbursement is based on whether patients are still responding after a month) and lymphoma treatment is $373,000.
The lymphoma price tag was set to match the competition — Gilead Science's Yescarta, which was approved last fall for the same relapsed lymphoma.
Novartis Oncology CEO Liz Barrett said in a statement that the European Commission's approval is "a transformational milestone for patients in Europe in need of new treatment options."
"This approval demonstrates the global impact of the therapies we developed in Philadelphia," Penn immunotherapy pioneer Carl June said in a statement.
Timing for Kymriah availability in each country "will depend on multiple factors" the company's statement said. "Training is already underway at key qualified treatment centers … and Novartis continues to collaborate with national health and reimbursement authorities across Europe."
The existing manufacturing plant is in Morris Plains, N.J. Novartis is pursuing expansion through agreements with Germany's Fraunhofer Institute and a French plant, CELLforCURE.
Fewer than 1,000 pediatric leukemia patients in the U.S. and Europe are candidates for Kymriah. An estimated 6,500 U.S. adults and more in Europe could use it to battle relapsed lymphoma.
In the first half of this year, Kymriah has had $28 million in sales. Novartis hopes the market will ultimately grow to $1 billion a year.