Rutgers enrolling children under age 5 for COVID bivalent vaccine trial
The Pediatric Clinical Research Center at Rutgers Robert Wood Johnson Medical School is recruiting participants for an international clinical trial.
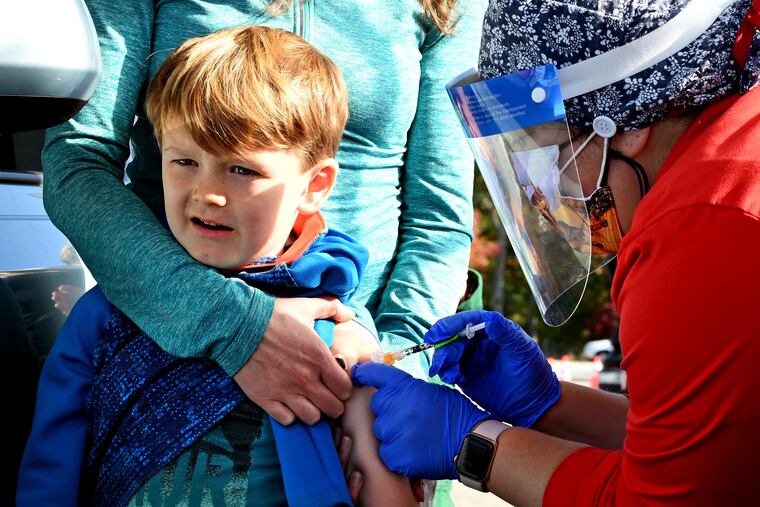
Philadelphia-area families with young children interested in the new bivalent COVID vaccine have an opportunity to get a shot not yet available for children under 5 years old.
The Pediatric Clinical Research Center at Rutgers Robert Wood Johnson Medical School is recruiting participants for an international clinical trial evaluating the bivalent COVID-19 vaccines effectiveness among young children.
The bivalent vaccine, which protects against highly contagious omicron COVID variants, was released for adults earlier this year and made available in October for children who are at least 5 years old. Young children and infants are currently eligible only for the original COVID vaccines.
Rutgers is participating in two of four sub-studies within the Pfizer-BioNTech trial:
Children ages 6 months through 23 months who have not received any COVID vaccine doses and will receive three doses (possibly a fourth) as part of the study.
Children ages 6 months through 4 years who have received three prior doses of the COVID vaccine and will receive the new bivalent as their fourth dose.
» READ MORE: The COVID vaccine boosters are now available for kids as young as 5 years old. Here’s what to know.
Throughout the study, participants will be closely monitored by doctors, with periodic clinical evaluations and blood tests. They will also be swabbed to test for COVID-19.
The bivalent boosters use the same mRNA platform that enables the body to build immunity to the virus that causes COVID-19. They are an updated version of the original vaccine, which has been proven safe for young children.
“The vaccines thus far have proven to be quite safe as millions of doses have been given to children. Hopefully the bivalent vaccines will help us get ahead of and protect us from the viral mutations,” co-lead investigator Simon Li, an associate professor of pediatrics at Rutgers Robert Wood Johnson Medical School, said in a statement.
To find out if your child is eligible, complete the online self-referral survey.