‘Skunkworks’ at Johnson & Johnson is rushing to develop a coronavirus vaccine
J&J set up the project two weeks ago when the virus began to look like a global threat.
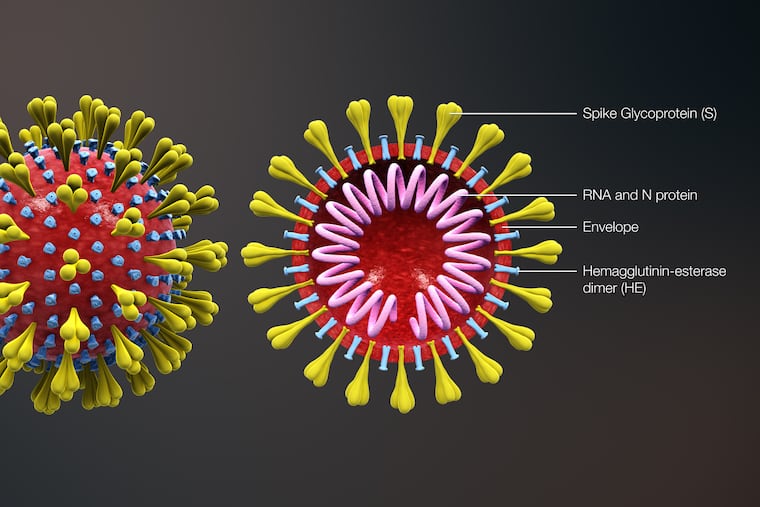
The chief scientific officer at Johnson & Johnson said the company began work two weeks ago on a vaccine to battle the deadly coronavirus outbreak centered in China.
“At the moment we think we can make a vaccine and bring it to humans in the next eight to 12 months,” said Paul Stoffels in an interview with The Inquirer. “It might be faster. We have to get to the point we know where it works in animals first.”
Stoffels said a Johnson & Johnson team started up a "skunkworks” to develop seven constructs for a vaccine as the Wuhan virus threatened to become a global crisis. Stoffels said the research effort, which began with about 15 people, “didn’t wait for [dedicated financial] support to kick it off. We’ll see as we go and find partners to collaborate to make that happen.”
China has confirmed more than 4,500 cases of the novel virus, which has caused more than 100 deaths, according to authorities. Most of the cases have been in the central city of Wuhan, where the illness first surfaced last month. More than 40 cases have been confirmed in other places with virtually all of them involving Chinese tourists.
» READ MORE: Philly-area company gets $9 million grant to develop vaccine for new Chinese coronavirus
Five cases of the Wuhan virus have been reported in the United States. There is also a suspected case in the Philadelphia suburbs.
Although eight to 12 months may sound glacially slow, pharmaceutical experts said the timeline was relatively rapid yet conservatively realistic.
Johnson & Johnson’s deep-pocketed researchers are using multiple platforms, or defined technological processes, that are allowing them to build and scale up new virus killers rapidly.
J&J and others used similar platforms to create an Ebola vaccine and deliver two million doses to West Africa in less than six months. The company also has used the platforms to create vaccines for HIV and Zika.
The company is conducting its Wuhan coronavirus research in Leiden, in the Netherlands. Stoffels spoke to The Inquirer from Leiden after addressing scientists at its labs.
Late last week, he spoke at the World Economic Forum in Davos, Switzerland, about the emerging coronavirus threat.
Stoffels said J&J is also screening a range of already existing medicines— both already in the market and in development — to see if they may be effective.
“The HIV protease inhibitors may be active on coronavirus,” he said. “The Chinese government already has asked for access to these medicines, but we need to prove that they work. That could be a few days to a few weeks.”
The company is donating 300 boxes of its HIV medication Prezcobix to the Shanghai Public Health Clinical Center, in hopes it may be effective against the virus, which is more formally called 2019-nCoR.
J&J’s announcement that it was gearing up to battle the virus comes a week after Inovio Pharmaceuticals Inc. in Plymouth Meeting said it had received a grant of up to $9 million to rapidly develop a vaccine for the Wuhan virus. Inovio is collaborating with the Wistar Institute in Philadelphia.
Other drugmakers also are searching for protective meds. According to Endpoints, a pharmaceutical-industry intelligence newsletter, Gilead Sciences is seeking to repurpose remdesivir -- an antiviral developed to treat Ebola. Vir Biotechnology is working to determine if its previously identified anti-coronavirus monoclonal antibodies can neutralize the virus.
Stoffels said he hopes the virus is contained and the threat of a pandemic dies down before a vaccine is needed.
“If the epidemic is over in a few months, we are all happy,” he said. “If it’s not and it expands, then we have gained many months of time to come up with a vaccine. We’ll be able to protect more people from getting the disease. That’s what we do.”