Abbott coronavirus test used by White House may be no better than a coin toss, study finds
The U.S. needs tests that diagnose coronavirus quickly, conveniently, but most of all, accurately
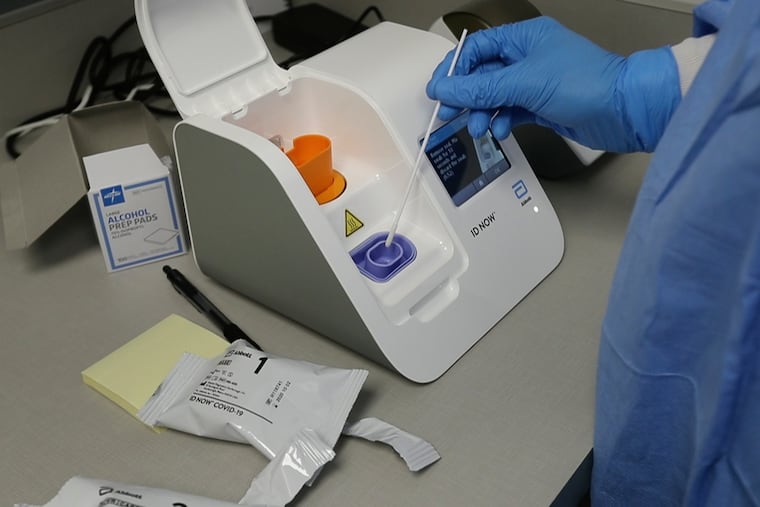
The Abbott Laboratories rapid-diagnostic test touted by President Donald Trump and used to monitor White House staff missed almost half of the coronavirus cases detected by a competing company’s test, according to a preliminary study.
The study by New York University researchers, published by Bioxriv but not yet vetted by other experts, collected 101 nasal swabs and 101 nasopharyngeal, or back-of-the-throat, swabs from the same COVID-19 patients to compare Abbott’s testing machine with rival Cepheid’s.
Abbott’s test missed a third of the samples detected as positive by Cepheid when using nasopharyngeal swabs. Abbott missed even more — 48% — when using nasal swabs.
Abbott’s testing platform does have advantages. The machine is small, can be used at drive-through swab collection sites, and gives results as fast as five minutes.
In contrast, Cepheid’s platform is used in high-complexity labs and results take about 45 minutes. The NYU researchers have been using Cepheid’s platform and one made by Roche that has a turnaround time of 3.5 hours.
“In search for a platform with shorter turnaround time, we sought to evaluate the recently released Abbott ID NOW COVID-19 assay,” wrote the researchers, led by pathologist Maria E. Aguero-Rosenfeld, director of the clinical laboratories at NYU Langone Health System.
In a statement Wednesday, Abbott defended its test, and said the NYU results “were not consistent with other studies” and “we have many questions for the study authors.”
“While no test is perfect, Abbott’s ID NOW is delivering reliable results when and where they’re needed most. The test is performing as expected by the more than 1,000 sites using ID NOW for COVID-19,” the statement said.
Reliability has been a problem with coronavirus testing, starting with the molecular diagnostic test kit made and distributed by the Centers for Disease Control and Prevention. Now, hundreds of commercial tests have been given emergency authorization by the Food and Drug Administration, but none were required to be validated in real-world clinical use – just in laboratories using known samples.
Even tests that perform almost perfectly in labs may miss lots of cases in the real world because only an estimated 3% to 5% of the population has been exposed to the virus. That low prevalence increases the probability that a result will be wrong – either missing signs of infection, or mistakenly finding signs that aren’t there.
Wrong results, in turn, can have terrible consequences.
“Accurate diagnostic testing and subsequent isolation of sick individuals is crucial to mitigating continued infectious transmission,” the NYU researchers concluded.