A Temple cardiologist was frustrated with devices to treat blood clots in lungs, so he invented a new one
The device to treat pulmonary embolism got approval from the Food and Drug Administration.
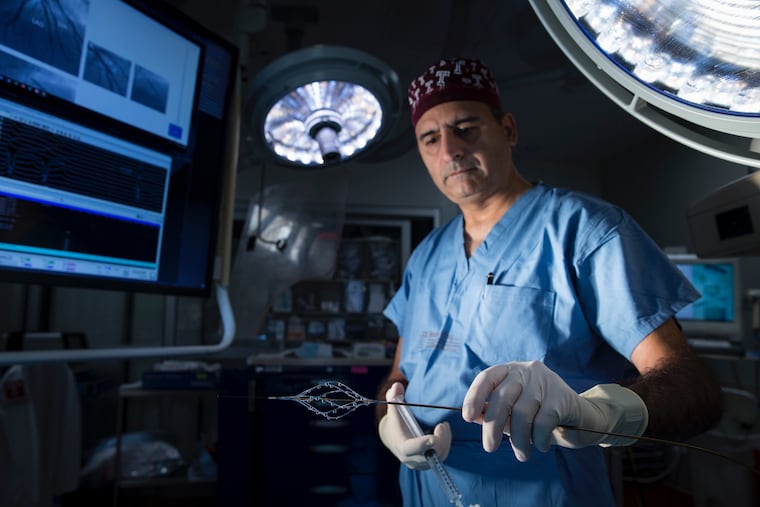
Riyaz Bashir was frustrated with the tools he had to treat blood clots in the lungs. So the Temple University Hospital cardiologist invented a new device to treat the nation’s third-leading cardiovascular cause of death.
Last month, the Food and Drug Administration approved his invention. Bashir believes the device is a significant milestone in the treatment of pulmonary embolism, which occurs when a blood clot gets stuck in one of the arteries in the lung. Blood then can’t reach the oxygen it needs to carry to the heart, brain, and the rest of the body.
“Hopefully it will benefit a lot of patients, not just in this country but around the globe,” Bashir said.
» READ MORE: Many Americans wrongly assume they understand what normal blood pressure is — and that false confidence can be deadly
The device, called a Bashir endovascular catheter, was developed through a collaboration between Temple and Thrombolex Inc., a Bucks County company that will develop and sell it.
Bashir, a cofounder of Thrombolex, said existing devices were originally intended to unclog blood vessels in the heart or legs. The arteries in the lung are much larger, and so are the clots that get stuck in them.
Because the clots in the lungs are so large, it takes many hours for existing tools to work. They rely on enough blood passing through the blockage to help it dissolve. To expedite the process, physicians give high doses of medicine that increase the risk of bleeding elsewhere.
The newly approved device aims to address all of those issues.
A clot-fighting spinner
Bashir’s invention, involving a flexible tube called a catheter, looks like the medical equivalent of a children’s spin toy.
A cardiologist inserts the device through a vein in the groin or neck and guides it to the clogged artery in the lung. Then it penetrates the blockage. Six small tubes with holes ring the catheter. Once in the clot, these tubes spin, creating a tunnel that allows blood to pass through.
The process spurs the blood’s clot-dissolving chemicals to start doing their job. In addition, medication is sprayed out of the little holes on the device’s tubes. This allows physicians to use lower doses of medication, reducing the risk of internal bleeding.
Procedures using this device takes about 15 minutes to complete.
» READ MORE: The FDA just approved one of the first drugs for ALS. Some raise questions, but this Penn patient has no doubt.
In a study published in December, Bashir and his coauthors showed data from 109 patients treated using the device. Overall, it worked to clear their blockages faster and with less clot-dissolving medicine when compared to other available techniques on the market. The rate of bleeding and other adverse events was also very low.
“This is an amazing result,” Bashir said of the research findings, published in the Journal of the American College of Cardiology: Cardiovascular Interventions.
Now with the FDA approval, the cardiologist wants to educate his peers about the new tool. He hopes that it will become common practice in treating lung clots.